Hypochlorous acid is a fascinating compound that plays a crucial role in chemistry, healthcare, and sanitation. At its core, hypochlorous acid is a weak acid with the chemical formula HOCl. This simple yet potent molecule is widely used for its disinfectant properties, making it indispensable in various industries. But what exactly is the formula of hypochlorous acid, and why is it so important? In this article, we’ll delve deep into the science behind hypochlorous acid, its applications, and its significance in our daily lives.
Understanding the formula of hypochlorous acid is essential for grasping its behavior and utility. Composed of one hydrogen atom, one chlorine atom, and one oxygen atom, its molecular structure is both simple and effective. This unique arrangement allows hypochlorous acid to exhibit powerful oxidizing properties, making it an excellent disinfectant. From purifying water to sanitizing medical equipment, the applications of this compound are vast and varied.
While hypochlorous acid might sound complex, its chemistry is rooted in fundamental principles. Whether you're a student, a professional, or simply someone curious about the world of chemicals, this guide will walk you through everything you need to know. We'll explore its chemical properties, biological relevance, and industrial uses, all while answering the burning question: What is the formula of hypochlorous acid?
Read also:William Nylander Siblings A Closer Look At Their Lives And Achievements
Table of Contents
- What is the Formula of Hypochlorous Acid?
- What Are the Chemical Properties of Hypochlorous Acid?
- How Does Hypochlorous Acid Work as a Disinfectant?
- Applications of Hypochlorous Acid in Daily Life
- Is Hypochlorous Acid Safe for Use?
- What Is the Environmental Impact of Hypochlorous Acid?
- How to Produce Hypochlorous Acid?
- Frequently Asked Questions About Hypochlorous Acid
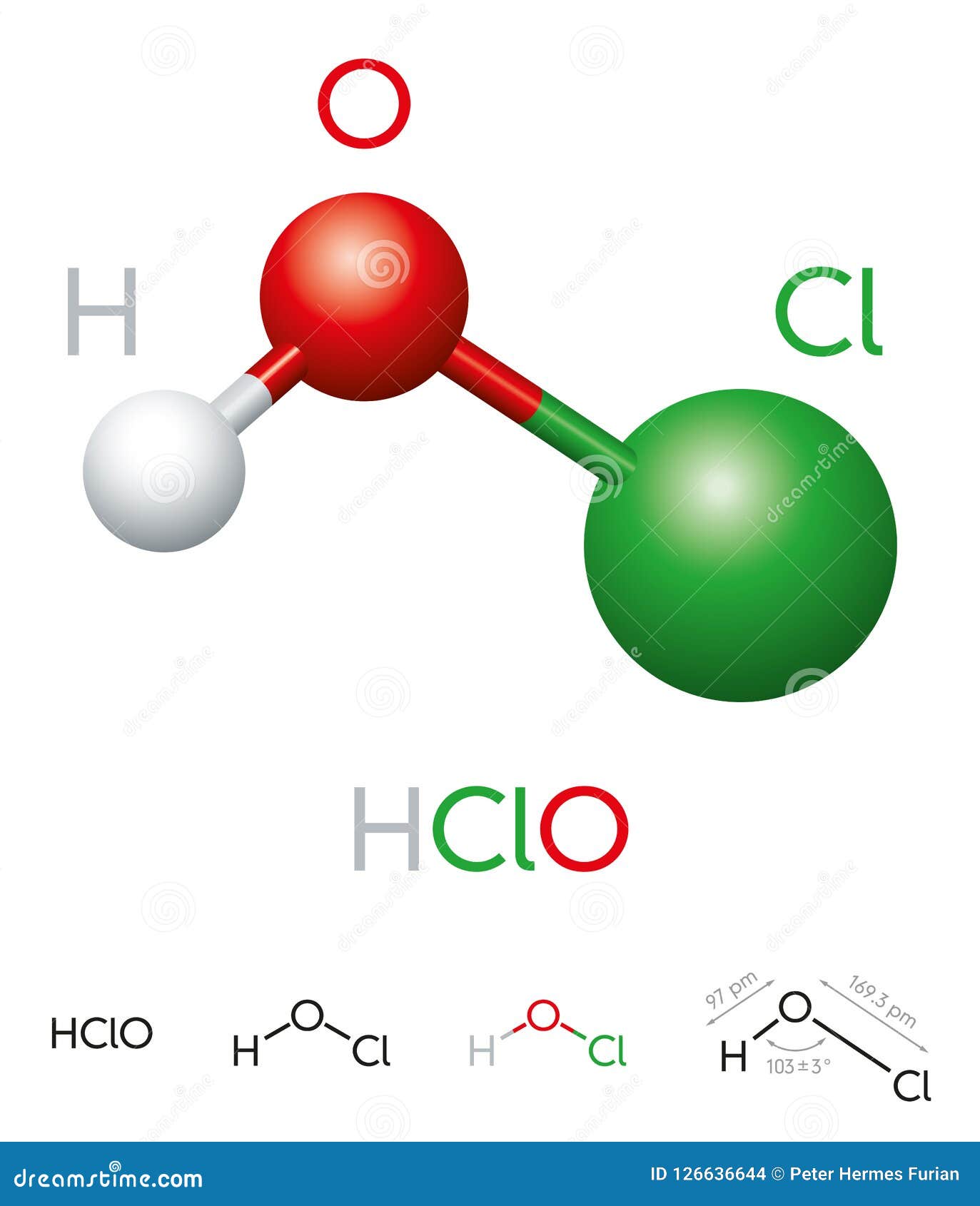
What is the Formula of Hypochlorous Acid?
At its core, hypochlorous acid is represented by the chemical formula HOCl. This formula indicates that the molecule consists of one hydrogen (H), one chlorine (Cl), and one oxygen (O) atom. The simplicity of its structure belies its powerful properties. Hypochlorous acid is a weak acid, meaning it does not completely dissociate in water. This characteristic is what makes it both effective and safe for various applications.
The formula HOCl is crucial for understanding how hypochlorous acid behaves in different environments. When dissolved in water, hypochlorous acid can exist in equilibrium with its conjugate base, hypochlorite ion (OCl⁻). This balance is influenced by factors such as pH levels. For instance, at lower pH levels, hypochlorous acid predominates, while at higher pH levels, the hypochlorite ion becomes more prevalent.
Understanding the formula of hypochlorous acid is also essential for its production and application. Whether synthesized in a laboratory or generated through electrolysis, the HOCl formula ensures that the compound retains its oxidizing properties. This makes it a versatile tool for disinfection, sanitation, and even wound care.
What Are the Chemical Properties of Hypochlorous Acid?
Is Hypochlorous Acid a Strong or Weak Acid?
Hypochlorous acid is classified as a weak acid. Unlike strong acids like hydrochloric acid (HCl), hypochlorous acid does not completely ionize in water. Instead, it partially dissociates, forming hypochlorite ions (OCl⁻) and hydrogen ions (H⁺). This partial dissociation is what gives hypochlorous acid its unique properties, making it effective yet safe for use in various applications.
What Makes Hypochlorous Acid a Powerful Oxidizing Agent?
The oxidizing power of hypochlorous acid stems from its ability to release oxygen atoms during chemical reactions. This property allows it to break down organic matter and kill microorganisms. For example, when hypochlorous acid comes into contact with bacteria, it oxidizes the cell walls, effectively neutralizing the threat. This makes it a popular choice for disinfectants and sanitizers.
How Does Hypochlorous Acid Work as a Disinfectant?
Hypochlorous acid's effectiveness as a disinfectant lies in its ability to penetrate microbial cell walls and disrupt vital processes. When applied to surfaces or used in water treatment, HOCl targets enzymes and proteins within bacteria and viruses, rendering them inactive. Its oxidizing properties make it highly effective against a wide range of pathogens, including E. coli, Salmonella, and even the influenza virus.
Read also:Who Is Apolo Ohnos Wife A Complete Guide To The Olympic Champions Life And Love
One of the reasons hypochlorous acid is preferred over other disinfectants is its safety profile. Unlike bleach, which can release harmful chlorine gas, hypochlorous acid is non-toxic and non-irritating when used correctly. This makes it suitable for use in sensitive environments, such as hospitals and food processing facilities.
Additionally, hypochlorous acid is environmentally friendly. It breaks down into harmless byproducts like salt and water, minimizing its ecological footprint. This is particularly important in industries where sustainability is a priority.
Applications of Hypochlorous Acid in Daily Life
Hypochlorous acid finds applications in a wide range of industries, from healthcare to agriculture. In healthcare, it is used to sanitize medical equipment, clean wounds, and disinfect surfaces. Its non-toxic nature makes it ideal for use in environments where patient safety is paramount.
In the food industry, hypochlorous acid is employed to sanitize fruits, vegetables, and meat products. It effectively eliminates harmful bacteria without leaving behind harmful residues, ensuring that food remains safe for consumption. Similarly, in agriculture, it is used to disinfect irrigation systems and control plant diseases.
Household applications of hypochlorous acid are also growing in popularity. From cleaning countertops to purifying water, this versatile compound is becoming a staple in eco-friendly cleaning products. Its ability to kill germs without posing health risks makes it a preferred choice for families and businesses alike.
Is Hypochlorous Acid Safe for Use?
Can Hypochlorous Acid Be Used on Skin?
Yes, hypochlorous acid is safe for use on skin when properly diluted. Its gentle yet effective nature makes it suitable for wound care and skincare products. Unlike harsh chemicals, hypochlorous acid does not irritate the skin, making it an excellent option for sensitive individuals.
What Are the Risks of Using Hypochlorous Acid?
While hypochlorous acid is generally safe, improper use can lead to risks. For instance, using highly concentrated solutions may cause skin irritation or respiratory issues. It’s essential to follow guidelines and use diluted forms of the compound to ensure safety.
What Is the Environmental Impact of Hypochlorous Acid?
Hypochlorous acid is considered environmentally friendly due to its biodegradability. Unlike other disinfectants that persist in the environment, hypochlorous acid breaks down into harmless substances like water and salt. This minimizes its impact on ecosystems and makes it a sustainable choice for industries focused on reducing their carbon footprint.
Moreover, hypochlorous acid does not contribute to the formation of harmful byproducts like trihalomethanes (THMs), which are often associated with chlorine-based disinfectants. This makes it a safer alternative for water treatment and sanitation processes.
How to Produce Hypochlorous Acid?
Hypochlorous acid can be produced through electrolysis of a saltwater solution. This process involves passing an electric current through a solution of sodium chloride (table salt) and water, resulting in the generation of hypochlorous acid. The method is simple, cost-effective, and scalable, making it ideal for both industrial and household applications.
Alternatively, hypochlorous acid can be synthesized in a laboratory by dissolving chlorine gas in water. However, this method is less common due to the potential hazards associated with handling chlorine gas.
Frequently Asked Questions About Hypochlorous Acid
What Is the Shelf Life of Hypochlorous Acid?
Hypochlorous acid is relatively unstable and can degrade over time. To maximize its shelf life, it should be stored in opaque containers away from direct sunlight. Proper storage can extend its usability for several months.
Can Hypochlorous Acid Replace Bleach?
Yes, hypochlorous acid can serve as a safer and more eco-friendly alternative to bleach. While both compounds are effective disinfectants, hypochlorous acid is non-toxic and non-corrosive, making it a better choice for sensitive applications.
Is Hypochlorous Acid Effective Against Viruses?
Absolutely! Hypochlorous acid is highly effective against a wide range of viruses, including enveloped viruses like the flu and non-enveloped viruses like norovirus. Its oxidizing properties make it a powerful tool for viral inactivation.
Conclusion
Hypochlorous acid, with the chemical formula HOCl, is a remarkable compound with a wide range of applications. From its role as a disinfectant to its use in healthcare and sanitation, understanding what is the formula of hypochlorous acid is key to appreciating its significance. Its safety, efficacy, and environmental friendliness make it a preferred choice for industries and households alike.
By exploring its properties, production methods, and applications, we gain a deeper understanding of why this compound is so valuable. Whether you're a student, a professional, or a curious reader, this guide has hopefully shed light on the fascinating world of hypochlorous acid.
For further reading, you can explore this external resource on the antimicrobial properties of hypochlorous acid.